As the product which increased the surface area of sub-structure of titanium tooth which inserted into the alveolar bone and extended the contact surface area with osseous tissue, and have various size and length to implant at the anterior and posterior or maxilla and mandible part according to the various condition of lossed bone.
ZEROS Implant is made of titanium and grafted in the maxillary or mandibular alveolar bone for surgical treatment. It plays the role of natural dental root. Refer to the manual or the catalogue or out website (www.zerosimplant.com) for detail. See the product label for the product code, specifications, manufacturing date, and expiration date.
Should be used in accordance with the general implant guide.
Following medical conditions may occur failure in cases when:
 Warnings
WarningsRead carefully instruction for use before use. The selection of inappropriate patients and operation methods can cause implant failure or loss of bone supporting the implant. ZEROS implants must not be used for purposes other than the recommended use and must not be remodeled. Implant mobility, bone less, and chronic infection can result in failure of the implant surgery.
The surgical technology of dental implant involves an expert, complex procedure. Formal training is required to perform implant surgery. Determine the local anatomy and suitability of the available bone for implant placement. Prepare the implant considering the expected situations and cautions. Visual inspections as well as panoramic and periapical radiographs are essential to determine anatomical landmarks, occlusal conditions, periodontal status, and the adequacy of the bone. Adequate radiographs, direct palpation, and visual inspection of the implant site are necessary prior to treatment, planning and use of ZEROS implant.
These Problems may occur after implantation (loss of implant stability, loss of prosthesis, etc). Deficient quality and quantity of remaining bone, infection, inferior oral hygiene or uncooperativeness of patient, implant mobility, partial deterioration of tissue, and improper position and arrangement of implants can cause instability.
 Surgical complications
Surgical complicationsThe implant procedure has risk, including localized swelling, dehiscence, tenderness of short duration, edema, hematoma, or bleeding. Numbness of the lower lip and chin region following lower jaw surgery, and of the tissue beside the nose following upper jaw surgery, is a possible side-effect of the surgery. Though it would most probably be of a temporary nature, in very rare cases, the numbness has been permanent. Gingival-mucosal(gum tissue) ulceration, tissue reaction, or infection may occur, but generally responds to local care.
and is ready to use. The sterilized product must be used in a sterilized environment with sterilized tools. If the packaged is damaged, or if the expiration date has passed, do not us the product. Expired or contaminated product must not be re-sterilized; they must be disposed of.
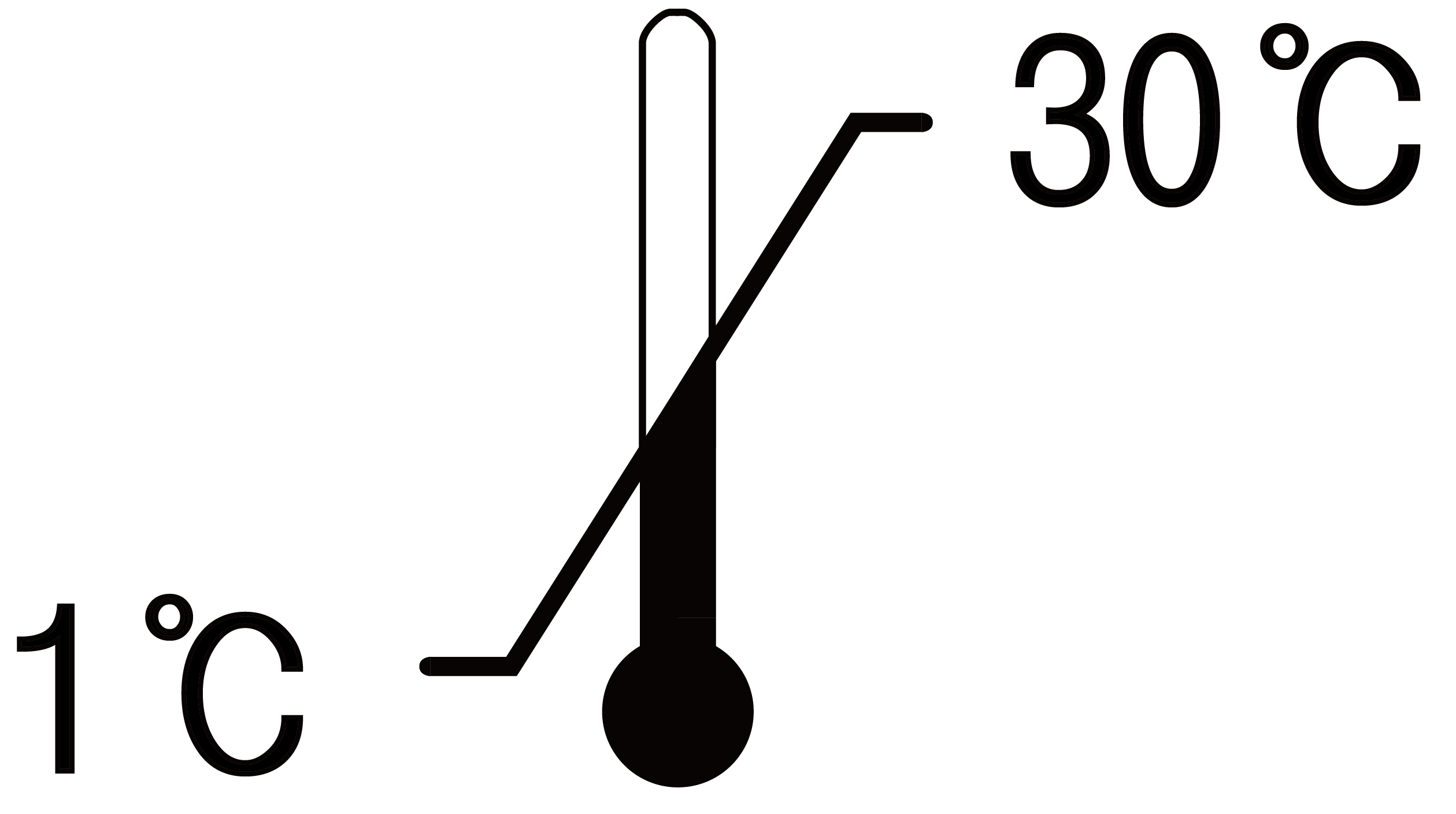
Keep the product in a dry place at room temperature(1℃~30℃). Keep away from direct sunlight. This product is valid for 3 years from the date of manufacture, please observe the validity date.
Determine the number, angles, and positions of the implants to be grafted considering a minimum gap of 3mm between implant. Local anesthesia is used; an anesthetic solution should be sufficient to anesthetize the periosteum surface. Conventional implant treatment should be applied. When using engine driver, the recommended speed is 15rpm, and maximum torque, 35Ncm. Once the maximum torque is reached, detach the driver mounted in the hand piece. Afterward, connect the torque wrench and perform grafting while rotating the hand-driver clockwise. It must be checked the information on length, diameter and depth for placement before use.
As the product which increased the surface area of sub-structure of titanium tooth which inserted into the alveolar bone and extended the contact surface area with osseous tissue, and have various size and length to implant at the anterior and posterior or maxilla and mandible part according to the various condition of lossed bone.
ZEROS Implant is a dental implant materials and consist of the Titanium. Refer to manual, catalog or our website (www.zerosimplant.com) for details. For the product code, specification, manufacturing date, and expiration date see the product label.
Should be used in accordance with the general implant guide.
The surgical technology of dental implantation requires an expert, it is a complex procedure which requires formal training to perform implantation.
It is important to look at the anatomy and suitability of the available bone for implant placement. Prepare the implant considering the expected situations and cautions. Visual inspection as well as panoramic and periapical radiographs are essential to determine anatomical landmarks, occlusal conditions, periodontal status, and adequacy of bone. Lateral cephalometric radiographs, CT scans, and tomograms may also be beneficial. In particular, the exact implant fixture can be assembled to prepare the abutment and the prosthetic components.
Contraindications include following, but are not limited to:
The Implant operation requires high accuracy and careful attention, we must try to minimize damage to the cell tissue and pay special attention to the temperature, surgical trauma, and/or removal of the source of contamination and infection.
The prosthetic structure is small; make sure it is neither swallowed nor inhaled by the patient. Angled abutments are not recommended for placement in the posterior region of the mouth due to limitations of implant strength. Stress distribution is especially important in implant operation as well as the fit of prosthesis and abutment on bridge, also the occlusal stability. Avoid using excessive force horizontally especially during immediate implantation. For the prosthesis whose substructure is made of gold alloy, gold should be used appropriately. Operate prosthesis after enough healing period.
Do not apply excessive stress on the replacement until the last prosthesis is placed.
 Side Effect
Side EffectThese Problems may occur after implantation (loss of implant stability, loss of prosthesis, etc). Deficient quality and quantity of remaining bone, infection, inferior oral hygiene or uncooperativeness of patient, implant mobility, partial deterioration of tissue, and improper position and arrangement of implants can cause instability.
 Warnings
WarningsRead carefully instruction for use before use. Using ZEROS Implant safely and effectively requires special training, since the surgical techniques involved in the dental implant operation are highly specialized and very complex. The selection of inappropriate patients and operation methods can cause implant failures or loss of bone supporting the implant. ZEROS Implant must not be used for purposes other than the recommended use. Dental implants must never be remodeled. If the implant is contaminated by the patient’s bodily fluids, it cannot be used in other patients. The ceramic abutment needs a special manufacturing process; the technician should be specifically trained in this process.
The unsterilized prosthetic components must be sterilized in an autoclave at 132℃ for 15 minutes before use. After the steam sterilization, the abutments should be dried for 15 minutes before use. This product is disposable medical device product in any case should not reuse.
Keep in cool(1℃~30℃) and dry place. Keep Out of direct sunlight. This product is valid for semi-permanent before open.
Surgical instrument which use for dental Implant surgery.
 Precaution
PrecautionShould be used by professional dentist, and forbid to use beyond of purpose. perform sterilization before the use after separate with other instrument. The professional dentist should perform the surgery after mast the use method. should careful handling the surgical instrument not hurt neighboring tissue. should replace it if have problem in appearance and after drop in the floor. and remove the foreign material after surgery using ultrasonic washing machine.
Please refer our home page(www.zerosimplant.com) and catalog or manual for the surgery procedure and detail product operating method and sequence.
Should use for decided usage only. must understand the product and surgical procedure before surgery. must check deform of appearance and scratch and damage of product.
The unsterilized prosthetic components must be sterilized in an autoclave at 132℃ for 15 minutes before use. After the steam sterilization, the abutments should be dried for 15 minutes before use. This product is disposable medical device product in any case should not reuse.
Remove the water after washing and storing in the inside of room which not have direct sun shine.
 |
 |
 |
 |
 |
|||||
| Representative | Caution | Date of manufacture | Manufacture | Do not reuse | |||||
 |
 |
 |
 |
 |
|||||
| Keep away from sunlight | Lot No. | Non sterile | Gama Sterilized | Use by date | |||||
 |
 |
 |
|||||||
| Temperature Limited | 0197 | Reference code | |||||||